Recent research by our group at the Technion – Israel Institute of Technology investigates the underlying chemistry behind plasma – assisted ammonia decomposition and oxidation. This parametric study explores the effects of varying the plasma and mixture conditions of ammonia fuels. A non-equilibrium nanosecond-pulsed high-frequency dielectric barrier discharge (DBD) plasma was employed in this work to crack ammonia, generating hydrogen and nitrogen.
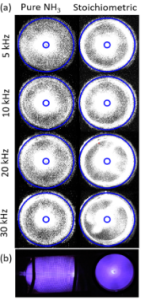
Ammonias high volumetric hydrogen density and combustible potential position it as a promising zero-carbon fuel source. However, drawbacks exist attributed to its poor combustion qualities relating to ignition resistance, narrow flammability range, and high NOx emissions. This study seeks to assess the feasibility of ammonia as a fuel source blended with hydrogen via partial dissociation and ammonia as a hydrogen vector via complete dissociation.
Species and Temperature Measurements
The rotational and vibrational temperatures of nitrogen species within the plasma were measured via OES, targeting the N2 second positive system. Since the N2(X) + e− → N2(C) + e− is fast and the dominating populating mechanism; the assumptions can be made that the 𝑁2(C) state follows the distribution of the 𝑁2(X) ground state, that rotational-translational equilibrium is fast, and thus the gas temperature is approximately the rotational temperature of the nitrogen species in the plasma. An in-house fitting software was used for quantification. This study examines the effects of varying the discharge parameters on the plasma temperature, which is a key contributor to the process of ammonia decomposition and oxidation, which is favored by high gas temperatures.
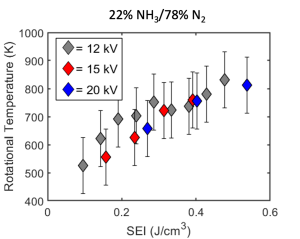
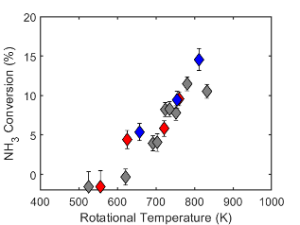
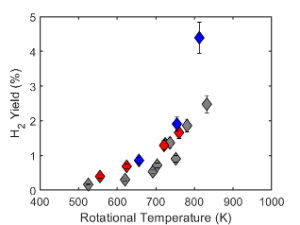
FTIR spectroscopy and GC-TCD measurements were performed on the exhaust gas to quantify ammonia conversion and hydrogen production. The specific energy input (SEI) is the main driver of the NH3 conversion and H2 yield, which is favored by achieving elevated gas temperatures. with a maximum H2 yield of 4.38%.
 Numerical Study
Numerical Study
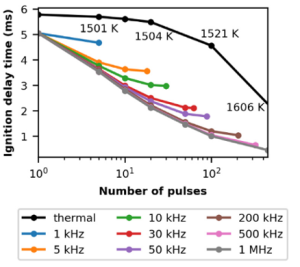 Alongside the experimental analysis of the ammonia plasma system, a numerical study investigates the plasma’s effects on ignition delay time (IDT) and reforming chemistry. By integrating the CHEMKIN combustion solver and the ZDPlaskin discharge solver in a zero-dimensional simulation, IDT was modeled to decrease by 40-60% with high pulse frequencies.
Alongside the experimental analysis of the ammonia plasma system, a numerical study investigates the plasma’s effects on ignition delay time (IDT) and reforming chemistry. By integrating the CHEMKIN combustion solver and the ZDPlaskin discharge solver in a zero-dimensional simulation, IDT was modeled to decrease by 40-60% with high pulse frequencies.



